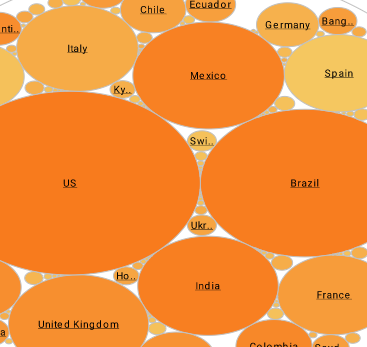
Pharmacometrics Software Example
Pharmacometrics is a multidisciplinary field at the intersection of pharmacology, mathematics, statistics, and computer science, aimed at quantifying and understanding the relationship between drug exposure, response, and patient characteristics. It utilizes mathematical and statistical models to analyze data from clinical trials, preclinical studies, and real-world settings to optimize drug therapy.
Pharmacometricians develop models to predict drug concentrations in the body, understand how drugs interact with biological systems, and tailor dosing regimens for individual patients or specific populations.
By integrating diverse sources of data and employing sophisticated modeling techniques, pharmacometrics plays a pivotal role in drug development, regulatory decision-making, and personalized medicine, ultimately enhancing patient care and therapeutic outcomes.
| #1 Ranking: Read how InetSoft was rated #1 for user adoption in G2's user survey-based index | Read More |
What Is Displayed in a Pharmacometrics Data Visualization?
In pharmacometrics data visualization, a wide array of information can be displayed, depending on the specific objectives of the analysis and the complexity of the model being used. These visualizations often present graphical representations of pharmacokinetic (PK) and pharmacodynamic (PD) data, including drug concentration-time profiles, dose-response relationships, and biomarker dynamics. Additionally, pharmacometrics visualizations may illustrate model diagnostics, such as goodness-of-fit plots, to assess how well the model describes the observed data.
Population analyses may involve displaying parameter estimates and their uncertainty across different subgroups or covariate values. Moreover, sensitivity analyses and simulations can be visually depicted to explore the impact of various factors on drug exposure and response. Overall, pharmacometrics data visualization serves as a powerful tool for summarizing complex model outputs, aiding in model evaluation, interpretation, and communication to stakeholders involved in drug development and clinical decision-making.
Drug Concentration-time Profiles
Drug concentration-time profiles depict the change in drug concentration in the body over time following administration of a medication. These profiles are fundamental in pharmacokinetics, the study of how drugs are absorbed, distributed, metabolized, and excreted by the body. The concentration-time curve typically displays the drug concentration on the y-axis (often in units like milligrams per liter or nanograms per milliliter) and time on the x-axis (usually in hours or minutes). The shape of the curve reflects the drug's pharmacokinetic properties, including absorption, distribution, metabolism, and elimination processes.
For example, the curve might show a rapid increase in concentration during the absorption phase, followed by a plateau or decline during distribution and elimination phases. Understanding drug concentration-time profiles is crucial for determining optimal dosing regimens, assessing drug efficacy, and predicting potential side effects or toxicity. Pharmacokinetic modeling techniques can be applied to interpret these profiles quantitatively and derive important pharmacokinetic parameters such as clearance, volume of distribution, and half-life, which inform drug dosing and therapeutic monitoring strategies.
Dose-response Relationships
Dose-response relationships elucidate the relationship between the dose of a drug or treatment and its effect on biological systems. This relationship is fundamental in pharmacology and toxicology, as it helps understand how varying doses of a drug impact physiological responses or clinical outcomes. Typically displayed graphically, dose-response curves illustrate the magnitude of the response (e.g., therapeutic effect, side effects, toxicity) as a function of increasing doses of the drug. The shape of the curve can vary depending on factors such as the drug's mechanism of action, receptor binding kinetics, and individual variability in response.
Common dose-response curve shapes include sigmoidal (S-shaped) curves, where the response increases gradually before reaching a plateau, or hyperbolic curves, where the response increases rapidly at low doses before leveling off. Parameters such as the maximum effect (Emax), potency (e.g., EC50, ED50), and slope of the curve provide valuable insights into the drug's pharmacodynamics and inform dosing strategies, therapeutic index calculations, and risk-benefit assessments. Understanding dose-response relationships is crucial for optimizing drug efficacy while minimizing adverse effects, thus guiding drug development, clinical practice, and regulatory decision-making.
Biomarker Dynamics
Biomarker dynamics refer to the changes in biomarker levels over time in response to various stimuli, interventions, or disease processes. Biomarkers are measurable indicators of biological processes, physiological states, or pharmacological responses, and they can include molecules such as proteins, nucleic acids, metabolites, or cellular structures. The dynamics of biomarkers can provide valuable insights into the underlying mechanisms of disease progression, treatment efficacy, and patient response to therapy. For example, in cancer research, biomarker dynamics might involve monitoring changes in tumor marker levels in response to chemotherapy or immunotherapy, helping to assess treatment response and predict patient outcomes.
Similarly, in pharmacokinetic/pharmacodynamic (PK/PD) modeling, biomarker dynamics may be used to characterize the relationship between drug exposure and the pharmacological effect, aiding in dose selection and optimization of therapeutic regimens. Biomarker dynamics can be visualized graphically over time, allowing researchers and clinicians to track trends, identify patterns, and make informed decisions regarding patient care and drug development strategies. Understanding biomarker dynamics is essential for advancing personalized medicine, improving disease management, and developing novel therapeutic interventions.
 |
Learn about the top 10 features of embedded business intelligence. |
What Features Are Found in Pharmacometrics Software?
Pharmacometrics software typically encompasses a diverse range of features tailored to support various aspects of pharmacometric analysis, modeling, and simulation. Some key features commonly found in pharmacometrics software include:
-
Model Development and Analysis: Pharmacometrics software facilitates the development and analysis of mathematical models to describe drug pharmacokinetics (PK) and pharmacodynamics (PD). It provides tools for model building, parameter estimation, model diagnostics, and visualization of model outputs.
-
Population Pharmacokinetics (PopPK) and Pharmacodynamics (PopPD): These software platforms enable population-based analyses to characterize variability in drug response among individuals or patient populations. They allow for the estimation of population pharmacokinetic and pharmacodynamic parameters and the exploration of covariate effects.
-
Nonlinear Mixed Effects Modeling (NLMEM): Pharmacometrics software often includes advanced algorithms and techniques for nonlinear mixed effects modeling, which is particularly useful for analyzing longitudinal data and accounting for inter-individual variability in PK/PD parameters.
-
Model Simulation and Prediction: Users can simulate drug concentration-time profiles, dose-response relationships, and other pharmacological endpoints based on developed models. This feature helps in predicting drug behavior under different dosing regimens, patient populations, or scenarios.
-
Covariate Analysis: Pharmacometrics software facilitates the identification and evaluation of covariates that influence drug pharmacokinetics or pharmacodynamics. It allows for the assessment of demographic, clinical, genetic, or environmental factors that may affect drug response.
-
Model Validation and Optimization: These tools enable the validation of developed models against observed data and the optimization of model structures and parameters to improve predictive performance and generalizability.
-
Regulatory Compliance: Pharmacometrics software may include features to ensure compliance with regulatory guidelines and standards for drug development, such as those set forth by regulatory agencies like the FDA (Food and Drug Administration) or EMA (European Medicines Agency).
-
Integration and Interoperability: Many pharmacometrics software platforms support data integration from various sources, including clinical trials, electronic health records, and preclinical studies. They may also offer interoperability with other software tools for data management, statistical analysis, and visualization.
-
User-Friendly Interface and Workflow Automation: These software solutions often feature user-friendly interfaces with graphical user interfaces (GUIs) and workflow automation capabilities to streamline the modeling process and enhance user productivity.
-
Education and Training Resources: Some pharmacometrics software providers offer educational resources, tutorials, and training programs to support users in learning and mastering pharmacometric modeling techniques and software functionalities.
 |
Read how InetSoft saves money and resources with deployment flexibility. |
Covariate Analysis
Covariate analysis in pharmacometrics involves the identification and evaluation of factors, known as covariates, that may influence drug pharmacokinetics (PK) or pharmacodynamics (PD) within a population. Covariates can be demographic characteristics (e.g., age, sex), clinical parameters (e.g., weight, renal function), genetic factors (e.g., polymorphisms), or environmental variables (e.g., smoking status). The goal of covariate analysis is to understand how these factors contribute to variability in drug response among individuals and to incorporate this knowledge into pharmacometric models to improve their predictive accuracy and generalizability.
The process of covariate analysis typically involves several steps:
-
Identification of Covariates: Researchers explore potential covariates that are biologically plausible or have been previously associated with variability in drug response. This may involve reviewing literature, conducting exploratory data analysis, or using prior knowledge of the drug's pharmacology.
-
Covariate Screening: Statistical methods are applied to screen covariates for their association with PK/PD parameters. Common approaches include univariate analysis, where each covariate is tested individually, and multivariate analysis, where multiple covariates are evaluated simultaneously while considering their interrelationships.
-
Covariate Model Building: Covariate analysis is integrated into the modeling process to identify significant covariates and quantify their effects on PK/PD parameters. This may involve developing mathematical models that describe how covariates influence model parameters, such as clearance, volume of distribution, or drug potency.
-
Model Selection and Validation: Models incorporating covariates are compared to simpler models without covariates using statistical criteria and diagnostic tools to assess goodness-of-fit, predictive performance, and clinical relevance. Models are validated internally and, if possible, externally using independent datasets.
-
Clinical Interpretation: The final covariate model is interpreted to understand the clinical implications of covariate effects on drug dosing, efficacy, and safety. This may involve identifying patient subpopulations with altered drug response due to specific covariates and informing personalized dosing recommendations.
 |
Read the top 10 reasons for selecting InetSoft as your BI partner. |
More Articles Related to Pharmaceutical Analytics
Drug Launch Analytics - The second use case that we're going to explore is really focused around drug launches and being able to maximize what that launch experience looks like. The key question that we're going to go ahead and shine a light on is how a pharmaceutical company might really be able to understand some of the early adoption related to their drug in the effort of being able to maximize their sales and being able to maximize their marketing launch as well...
Pharmaceutical Claims Data Analysis - Then there is a lot of great intelligence on physician groups and physicians operating in market. That includes 150,000 or so physician group profiles and intelligence on over 1.6 million healthcare professionals and physicians. Some of the key data that is tracked on all of these different types of healthcare providers really ranges from affiliations and the relationships that they have with other providers and market, plus some of the key executives and their contact information of staff each one of these providers...
Pharmaceutical Testing Dashboard Utility - InetSoft's pharmaceutical testing analytics provide a means to measure and evaluate the performance of drug testing trials. Researchers can assess the efficacy and safety of the drug candidate, monitor patient recruitment rates, track protocol adherence, and evaluate the overall progress of the trial. This information helps in optimizing trial design and resource allocation. InetSoft's mashup driven analytics...
Physician Drug Adoption Rates - Beyond the value of analyzing physician drug adoption rates, you can think about the kind of the affiliation structures that we've talked a little bit about that really helped to inform what your drug launch strategies should be based on, and how loosely or tightly aligned a doctor may be within our particular system. You also have the contact information for physicians whether that'd be the emails that we have on hand, the addresses...